
Exentec Biopharma Solutions (EBPS) is an industry leading provider of high quality biopharm process equipment skids with an installed base exceeding 1000 systems. We design (process, mechanical, electrical, controls, and software), and fabricate in our own shop, to offer GMP ready solutions for our clients. EBPS is best known for the manufacture of complex biopharmaceutical processing skids including but not limited to tangential flow filtration, single and multicolumn chromatography, inline buffer dilution, oligonucleotide, and mRNA processing systems. We support our clients process equipment needs from pilot-scale to high volume manufacturing.
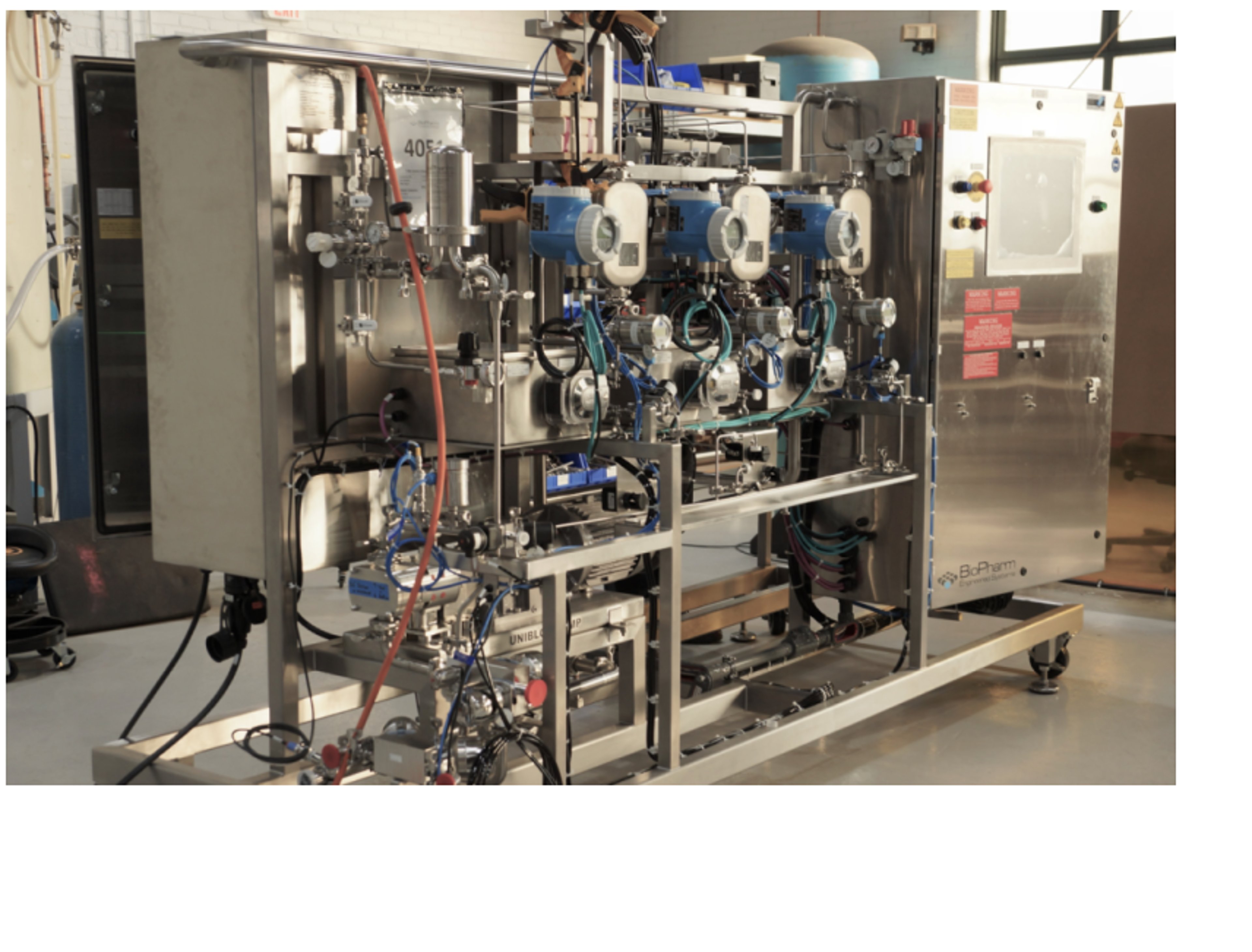
Custom Process Equipment
We provide specialized engineering, design, manufacturing and support services for a complete range of custom process separation and fluid handling system solutions including:
- Pre & post viral production trains
- Bioreactor / fermenter skids
- Inline dilution skids
- Single & multicolumn chromatography
- UF/DF/MF/SPTFF filtration skids
- mRNA systems (LNP T-Mix/Chrom/TFF)
- And more...

Design & Fabrication Services
Whether you are bringing a new product to market or working to increase plant efficiency and scale, our project management expertise enables you to bring your ideas to reality.
- Mechanical/electrical/process/controls Engineering
- Project management
- Mechanical design (3D Creo Pro E)
- Mechanical fabrication (ASME BPE/cGMP)
- Electrical panel fabrication (UL508A)
- Software programming
- Commissioning/qualification/verification

Custom Facility & Suite Support
Exentec Biopharma Solutions understands that no two projects are alike and that unique projects often require unique solutions to meet manufacturing/facility needs. We offer for example:
- Custom fabricated components
- Spooling, ported valves, tank trim
- Custom carts, lifts, funnels, drip pans and frames
- Component modification and repair
- Metal finishing & repairs
- Custom filter holders / housings
- Transfer & utility panels / stations

Process Solutions
Our R&D through manufacturing experience gives us an understanding of how a facility needs to accommodate technologies as they ramp from idea to full-blown operation – including how to integrate and protect multiple tenant types, predicting increasing infrastructure requirements, and designing sufficient flexibility.
- Project life cycle support
- Clean utility expertise
- Engineering services
- Staff augmentation services
- Life science expertise
- Process equipment expertise